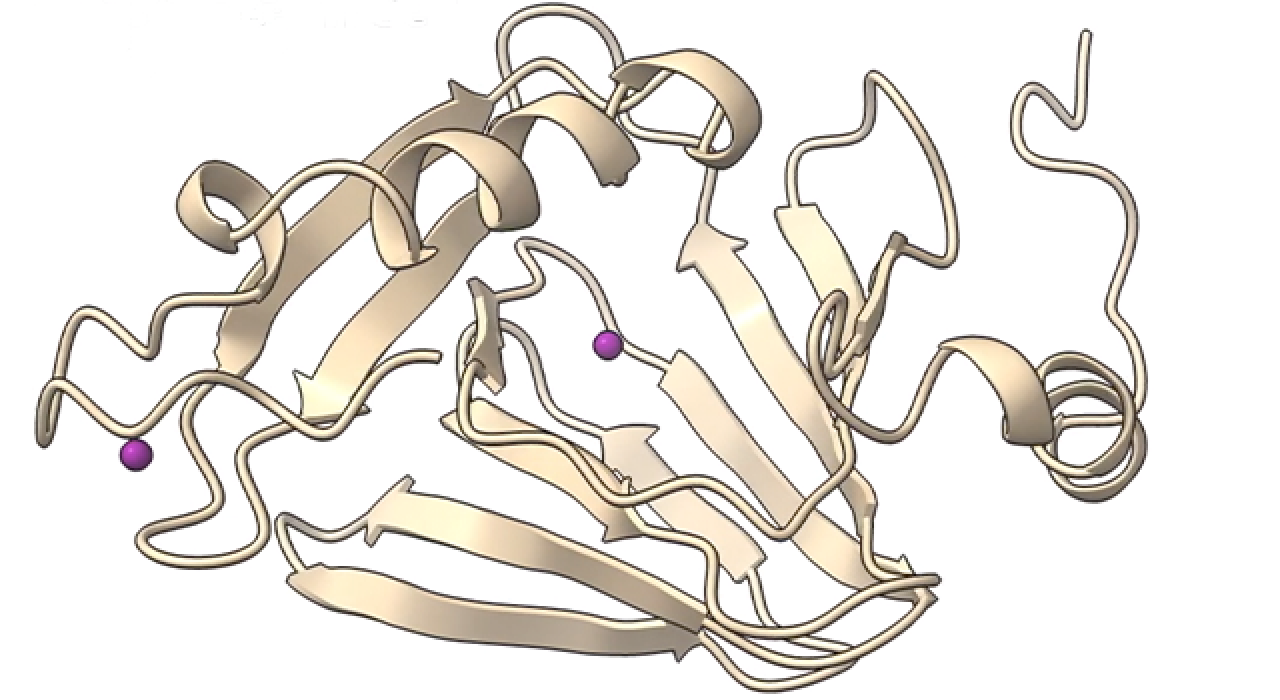
SEPTEMBER 3, 2020 — UTSA researchers have discovered how 3-hydroxyanthranilate-3,4-dioxygenase, an enzyme known as HAO that is involved in the breakdown of tryptophan and the biosynthesis of nicotinamide adenine dinucleotide, prevents the overproduction of quinolinic acid, which is associated with neurodegenerative diseases.
By combining single-crystal spectroscopic and X-ray diffraction studies, the researchers have for the first time identified seven of HAO’s catalytic intermediates. This important discovery will enable scientists to target the tryptophan-kynurenine pathway, or KP, as a therapeutic for neurodegenerative diseases and cancer.
“From a medical perspective, the HAO enzymatic mechanism is the main branching point for the KP pathway. When it’s hijacked by tumor cells, nicotinamide adenine dinucleotide production is disrupted and quinolinic acid can be overproduced by the body. This overproduction can lead to many diseases, including depressive behavior and neuropsychiatric disorders,” said Aimin Liu, professor and Lutcher Brown Distinguished Chair in Biochemistry in UTSA’s Department of Chemistry.
“This discovery is a game changer for an important branch of the [metabolic pathway].”
Enzymes are proteins that catalyze chemical reactions in the body. Since they work rapidly, it is difficult for researchers to study how they facilitate reactions. To overcome this challenge, the UTSA chemistry researchers crystallized HAO and conducted the chemical reaction in crystals.
In its crystalline state, HAO performs its chemistry over 10,000 times slower than in solution, making it possible for researchers to identify seven individual catalytic intermediates. By using X-ray diffraction, the researchers were able to determine the atomic structure of each intermediate. Solving the atomic structure of seven intermediates provided a detailed view of the enzyme’s chemistry.
HAO belongs to the KP metabolic pathway, which breaks down tryptophan for use in the biosynthesis of nicotinamide adenine dinucleotide (an essential nutrient) or for energy production. The KP is also used as a checkpoint to restrict the body’s immune response. Cancer cells overexpress KP enzymes to bypass and block immune T-cell proliferation, eventually inducing T-cell death. As a consequence, cancers can evade immune surveillance and increase tumor cell migratory capacity. Hence, selective and highly specific inhibition of KP enzymes, such as HAO, is a promising avenue to activate therapeutic antitumor immunity.
“This discovery is a game changer for an important branch of the KP. We finally have a complete look at [nicotinamide adenine dinucleotide] biosynthesis and can see all of its different branching points,” said Liu. “This underlying chemistry is going to be immense help to researchers who want to target the KP pathway for therapeutic treatments.”
The research leading to this discovery was performed in UTSA’s Department of Chemistry. The X-ray diffraction data were collected remotely at the Argonne National Laboratory Advanced Photon Source and the Stanford Synchrotron Radiation Lightsource.
⇒ Learn more about the Metalloprotein Research Lab at UTSA.
The Metalloprotein Research Laboratory at UTSA, led by Liu, investigates how biomolecules use metals to perform chemistry necessary for life. The latest study, “Observing 3-hydroxyanthranilate-3,4-dioxygenase in action through a crystalline lens,” has been published in The Proceedings of the National Academy of Science, a premier scientific journal.
The research team was led by Yifan Wang, a current Ph.D. research assistant in the Metalloprotein Research Laboratory; Kathy Fange Liu, a former Ph.D. student and now an assistant professor with the University of Pennsylvania’s Department of Biochemistry and Biophysics; Yu Yang, a former postdoctoral associate and currently an associate professor at Hubei University; and Ian Davis, a visiting chemistry scholar at UTSA—all and under the direction of Aimin Liu.